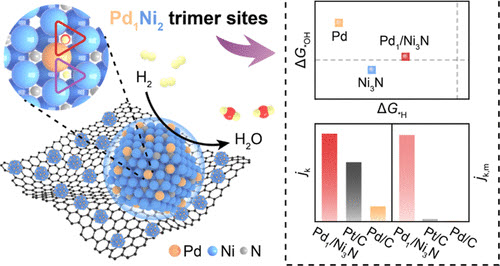
Anion-exchange membrane fuel cell (AEMFC) is a cost-effective hydrogen-to-electricity conversion technology under a zero-emission scenario. However, the sluggish kinetics of the anodic hydrogen oxidation reaction (HOR) impedes the commercial implementation of AEMFCs. Here, we develop a Pd single-atom-embedded Ni3N catalyst (Pd1/Ni3N) with unconventional Pd1Ni2 trimer sites to drive efficient and durable HOR in alkaline media. Integrating theoretical and experimental analyses, we demonstrate that dual Pd1Ni2 sites achieve a “*H on Pd1Ni2–HV + *OH on Pd1Ni2–HN” adsorption mode, effectively weakening the overstrong *H and *OH adsorptions on pristine Ni3N. Owing to the unique coordination mode and atomically dispersed catalytic sites, the resulting Pd1/Ni3N catalyst delivers a high intrinsic and mass activity together with excellent antioxidation capability and CO tolerance. Specifically, the HOR mass activity of Pd1/Ni3N reaches 7.54 A mgPd–1 at the overpotential of 50 mV. The AEMFC employing Pd1/Ni3N as the anode catalyst displays a high power density of 31.7 W mgPd–1 with an ultralow anode precious metal loading of only 0.023 mgPd cm–2. This study provides guidance for the design of high-performance alkaline HOR catalytic sites at the atomic level.
Article link: https://pubs.acs.org/doi/10.1021/jacs.4c17605





 Address
Address
 E-Mail
E-Mail
 Telephone
Telephone