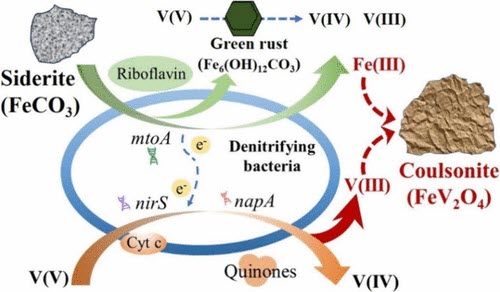
Vanadium (V) is a redox-sensitive metal with three valence states (+3, +4, +5) in Earth’s surficial environment. The microbially mediated transformation of hazardous vanadate [V(V)] plays a pivotal role in V geochemistry and detoxification. Tetravalent V [V(IV)] is the most common species resulting from V(V) bioreduction, but it is susceptible to reoxidation and release during redox fluctuation. This study demonstrated autotrophic V(V) reduction by widespread denitrifying bacteria Acidovorax sp. strain BoFeN1 and Pseudogulbenkiania sp. strain 2002 using siderite (FeCO3), a ubiquitous Fe(II)-bearing mineral on Earth. Advanced characterization techniques (TEM, XPS, XRD, and XANES) collectively confirmed the formation of trivalent V [V(III)] precipitates as coulsonite (FeV2O4), along with V(IV) as VO(OH)2. EXAFS and M?ssbauer spectroscopy further indicated the incorporation of V(III) into the magnetite lattice by substituting for Fe(III), resulting in more structurally stable FeV2O4 resistant to reoxidation. Cytochrome c, riboflavin, and quinones mediated electron transfers from siderite to V(V), while napA, nirS genes, and mtoA genes regulated V(V) reduction and Fe(II) oxidation. This study found that V(V) reduction in an aquifer was stimulated by the synergistic functions between the introduced denitrifying bacteria and indigenous communities with siderite supplementation, providing unique insights into the biogeochemical cycling of V and its bioremediation.
Article link: https://doi.org/10.1021/acs.est.4c11605





 Address
Address
 E-Mail
E-Mail
 Telephone
Telephone